Antibodies (Immunoglobulins)
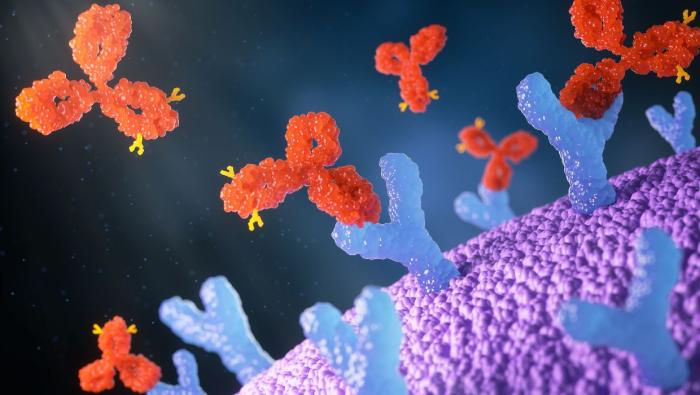
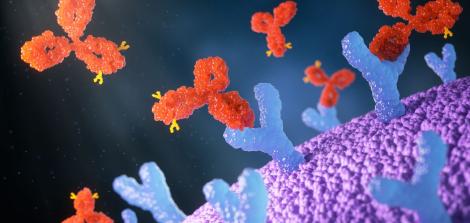
Antibodies are protein molecules that belong to the adaptive immune system. Their main role is to protect the body by recognizing, binding to, and marking specific foreign substances (antigens), such as proteins found on bacteria, viruses, toxins, or cancer cells.
How are antibodies produced, and what is their structure?
Antibodies are made by a type of white blood cell called B cells (B lymphocytes). After encountering an antigen, B cells transform into plasma cells, which produce and release antibodies tailored specifically to that antigen into the bloodstream.
All antibodies share a basic Y-shaped structure made of four protein chains. This structure includes two main regions:
The constant region (the “tail”) – This part determines the antibody’s class and allows it to interact with other immune cells, such as phagocytes, so the invader can be eliminated.
The variable region (the tips of the “Y”) – This is the antigen-binding site. This region is unique to each antibody and fits only one specific antigen, much like a key fitting a single lock.
What do antibodies do?
Antibodies help neutralize pathogens in several key ways. First, they can neutralize a threat directly: an antibody binds to a pathogen or toxin and prevents it from attaching to the body’s cells, blocking its ability to cause harm.
Second, they act as markers. Once an antibody coats an invader, it becomes easy for the immune system’s “clean-up crew,” the phagocytes, to recognize and engulf it.
Finally, antibodies help activate the complement system, a set of immune proteins that circulate in the blood as part of the innate immune system. When an antibody binds to an antigen, it triggers a chain reaction within the complement system that powerfully amplifies the immune response and leads to the destruction of the pathogen.
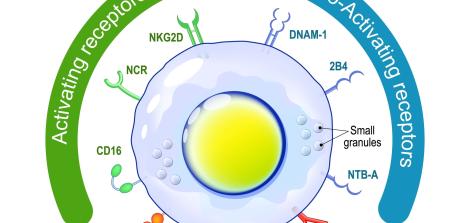
What are the main types of antibodies, and why are they important in blood tests?
There are five major classes of antibodies (immunoglobulins). The two most important for identifying infections are:
IgM – the first antibody to appear in an initial immune response. Its presence usually indicates a recent infection or a new exposure.
IgG – the most common and abundant antibody in the blood. It appears later, remains in the body for a long time, and reflects past exposure and immune memory (immunity).
Blood tests that measure antibodies, such as serological tests for viruses, can distinguish between these types and help determine the stage of an infection.
How are artificial antibodies used in modern medicine (immunotherapy)?
One of today’s central medical and research fields is the use of artificial antibodies, which are antibodies produced in the laboratory and designed for a specific task. These antibodies, called monoclonal antibodies, are a cornerstone of personalized medicine, especially in cancer treatment through immunotherapy, a method that strengthens the patient’s own immune system.
Cancer cells are able to “hide” from the immune system by sending signals that “switch off” immune cells and prevent them from attacking. Artificial antibodies, such as the drug Pembrolizumab (also known as Keytruda), bind to these checkpoint sites and block the shutting-down signals. In doing so, they release the brakes on the immune system and allow it to recognize and destroy the cancer cells on its own.
Last Updated Date : 31/12/2025