“It’s Only a Matter of Time Before We Find a Cure”: Groundbreaking Israeli Research Offers New Hope for Cancer Treatment
What causes immune cells to grow exhausted in their battle against cancer, and how can we help them recover? How is artificial intelligence accelerating medical research and helping turn cancer into a manageable chronic disease? And how do physical forces prevent immune cells from penetrating tumors? An interview with Professor Mira Barda-Saad of the Dangoor Center for Personalized Medicine on groundbreaking Israeli cancer research.
“A few decades ago, being diagnosed with blood cancer was almost a death sentence. Today, thank God, most types of these cancers can be cured, and some have even become chronic diseases treated for life,” says Professor Mira Barda-Saad of the Dangoor Center for Advanced Medicine at Bar-Ilan University. After helping to develop treatments for blood cancer, she is now working tirelessly to find a cure for pancreatic cancer. Will she succeed?
We met with Professor Barda-Saad, head of the Molecular and Applied Immunology Research Laboratory, to hear about the revolutionary therapeutic approaches she is developing and the new hope she offers millions of cancer patients worldwide.
Our immune system is often compared to an army that protects the body, trained to identify and destroy bacteria and viruses. Immunotherapy, the main field of research for Professor Mira Barda-Saad, is a medical approach that harnesses the body’s own immune system to fight disease, particularly cancer and autoimmune disorders. In these conditions, the immune system is either too weak to act or mistakenly turns against the body itself. While immunotherapy aims to boost immune activity against cancer cells or infections, in autoimmune diseases the opposite is required: the immune system must be suppressed to prevent it from attacking the body’s own tissues.
 “Cancer cells are far trickier than we think,” says Professor Mira Barda-Saad.
“Cancer cells are far trickier than we think,” says Professor Mira Barda-Saad.
If our bodies have such a powerful army within them, why doesn’t it fight cancer cells more quickly?
“Cancer cells are far trickier than we think,” explains Professor Mira Barda-Saad. “They know how to hold back our immune system. It’s been discovered that there are receptors on immune cells that cancer cells exploit to suppress them. Pharmaceutical companies and scientists are now working on developing antibodies that block these negative receptors, allowing the immune cells to fight the disease more effectively.”
What did you discover happens to the immune system when facing the cancer you studied?
“We found that in the presence of disease, immune cells reach a state of exhaustion. They simply become tired, and their anti-cancer activity drops dramatically. We wanted to understand what in their molecular mechanism was telling them, ‘Give up, you can’t handle this.’
We discovered several regulators inside the cells, special kinds of ‘switches’ that control cellular processes – that drive transcription. Transcription is the process by which a cell produces proteins. When the cell needs a specific protein, it first copies the relevant information and then uses that copy to build the protein.
When immune cells begin to wear out, and lose their strength, these internal regulators respond by working harder. It looks as if they’re trying to help and compensate for the weakness, but in reality, the opposite happens – the regulators produce proteins that actually paralyze the cell and prevent it from fighting.”
 A pancreatic tumor. Photo: Shutterstock
A pancreatic tumor. Photo: Shutterstock
“In our lab, we created agents that neutralize those transcription factors. When we injected them into mice, the agents penetrated the tumor environment, and we saw a response: the solid tumors shrank. We managed to disable the mechanism that was essentially telling the cells to rest, and the immune cells showed much stronger anti-cancer activity.”
From COVID-19 vaccines to cancer vaccines: the journey continues.
Impressive. Let’s talk about vaccines for a moment. What are the differences between cancer vaccines and vaccines for viruses such as COVID-19?
“The strategy of using mRNA and nanoparticles, an approach in which genetic instructions are injected into the body to tell cells to produce a specific protein, which the immune system then recognizes and learns to attack, originally began as an anti-cancer strategy. Then, the pandemic hit, and major pharmaceutical companies redirected their research toward developing COVID-19 vaccines. Now, that same research is being rapidly refocused on cancer, with remarkable success. This is, in fact, the same cellular manipulation we discussed earlier.
It’s essential to recognize the differences between viral vaccines and cancer vaccines. Viral vaccines focus on prevention, while cancer vaccines aim for treatment. Viral vaccines present a viral protein as an antigen, whereas cancer vaccines introduce tumor-specific antigens. Additionally, the immune system is typically healthy and needs training for viral vaccines, but is often suppressed or unresponsive in cancer. Therefore, cancer vaccines usually require combination with other immunotherapies to activate the immune system..”
Your previous research focused on blood cancer. What did you do there?
“We developed a treatment using a small molecule to combat blood cancer. This molecule weighs less than 500 daltons, making it billions of times smaller than a single cell. Because of its size, it can easily penetrate and slip into cells. When we injected it into blood cancer cells, we discovered that it targeted a protein responsible for cell division, migration, and tissue invasion, and managed to break it down. Once that protein was dismantled, those processes stopped. We demonstrated this in human blood cancer cells injected into mice, published the findings, and the study received significant international attention.”
Can’t you apply what you did to other types of cancer as well?
“Not all models are relevant to every type of cancer. In cases where we can, we do, but when we can’t, we have to learn an entirely new mechanism of action from scratch. Understanding that mechanism and figuring out how to target it isn’t simple. Right now, we’re focusing on pancreatic cancer and using lung cancer as a disease model.”
What else are you researching these days?
“We’re working extensively in an entirely new field, where we’ve come to understand that physical forces have a real impact on immune cells. These cells infiltrate tumor tissue to fight it, but when they encounter tissue that’s dense and compact, they retreat, exhausted. We discovered that tissue stiffness plays a critical role – when the tissue is softer, the cells can infiltrate more easily, reach the core, and fight effectively. So we’re now exploring ways to treat the tumor tissue itself, softening it to help immune cells penetrate and do their job.”
You’re one of many researchers working at the Dangoor Center for Personalized Medicine. What exactly makes this kind of treatment personalized?
“Personalized medicine plays a major role in these treatments because every patient has their own unique arsenal, including the receptors on their cell surfaces. Some people’s immune cells are like ‘serial killers,’ while others have cells that are less effective. We’re working to identify these differences, characterize each patient’s immune system, understand it, and tailor the treatment accordingly.”
Can this also be related to a person’s diet or physical activity?
“Yes, absolutely. We know for certain that autoimmune diseases are strongly influenced by genetics and stress. There’s no doubt that chronic hunger can affect the immune system. In other words, malnutrition harms both the production and the function of immune cells.”
Have you seen an increase in cancer cases as a result of the stress caused by the war?
“Usually, the effects appear a few years later, not immediately. Cancerous processes take time to develop and for a person to feel their impact. It can start with a small inflammation that gradually worsens. We already know there’s a problem because people are consuming more psychiatric drugs. What will this stress do to people in the long term? I assume we’ll understand more in the coming years. Let’s all hope the situation changes and that we can go back to being happy and care-free.”
Artificial Intelligence as a Partner in the Lab
As an Israeli researcher, do you find it more difficult to operate internationally these days?
“There is a boycott, and we can’t ignore it. It affects us – there’s been a significant decline in research support here in Israel, and some institutions actually forbid their faculty from collaborating with Israeli scientists.
Even so, we continue to work and publish. For example, our research on the small molecule was featured on the cover of The EMBO Journal, and we’ve appeared on the covers of other prestigious scientific journals as well.
Israeli scientists are still leading the way. We’ve just returned from the International Congress of Immunologists in Vienna, where we were invited to present our research. We remain highly competitive compared with research groups around the world. The situation doesn’t stop us from working; on the contrary, it makes us even stronger.”
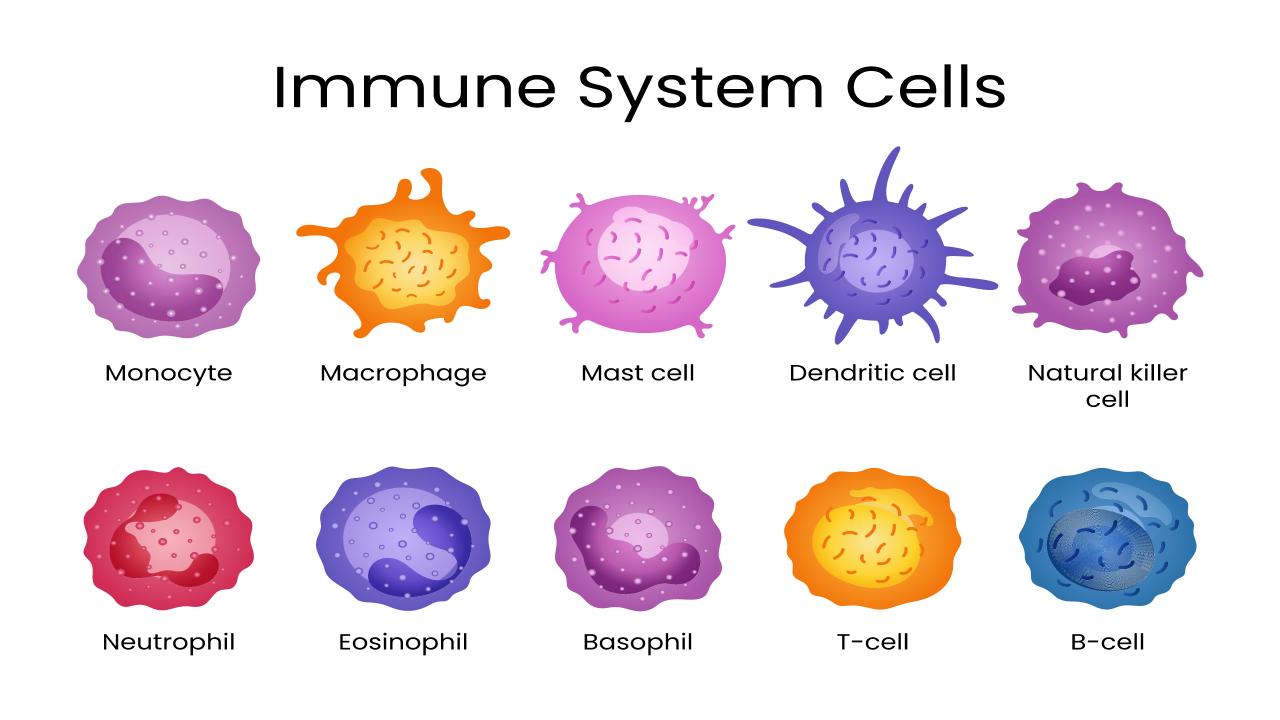 Types of immune system cells. Photo: Shutterstock
Types of immune system cells. Photo: Shutterstock
You’re developing approaches to treat cancer using artificial intelligence. How does AI help in cancer treatment?
“It’s incredibly helpful. This is truly a revolution, with many platforms and layers. I’ve studied it myself, and I encourage my students to gain as much knowledge in AI as possible and to use it to advance research in a faster and more reliable way. But they also need to verify and explore things independently, not rely solely on artificial intelligence. In our lab, we have a computational biology division, a subteam dedicated to working with innovative biological AI tools.”
Can you give an example of how you use these biological AI tools?
“We use artificial intelligence to identify small molecules that can eliminate solid tumors. We scan libraries containing hundreds of thousands of molecules to find those capable of neutralizing harmful factors. Just as most blood cancers now have effective treatments, I believe the same will happen with solid tumors. It’s only a matter of time. I don’t know how long it will take, but we’re all making every effort to get there.”
Does your research have applications even before reaching the drug development stage? What is the potential of your studies for developing treatments for other diseases you haven’t yet explored?
“The potential is huge, absolutely. When I was in Vienna, I felt like a kid in a giant toy store and realized, for example, that we’re getting quite close to finding a solution for allergies, which are among the most troublesome conditions out there. Many of the strategies developed for cancer treatment have been adopted by researchers studying chronic inflammatory diseases. The focus on these diseases comes from the understanding that inflammation can lead to cancer, so we take a few steps back and deal with inflammation early on to prevent the need for cancer treatment later.”
Based on your multidisciplinary background, how do collaborations between researchers from different fields contribute to the success of your studies?
“Today, no research is single-disciplinary. That’s a fact. Anyone who thinks they can sit alone and conduct research won’t succeed. Most studies today are multidisciplinary. We’ve collaborated with scientists from different fields and with clinicians to strengthen our research. For example, we’re now working with microbiologists, because we’ve learned that the microbiome has a real influence on the immune system.”
The microbiome refers to the bacteria in the gut?
“Exactly. They shape the immune system, which is why we’re studying them too. That’s the beauty of science; we keep learning more every day and stay flexible in light of what we discover. A good scientist, beyond being endlessly curious, must also be adaptable, both in thinking and in strategy, to adjust to the new information they encounter.”
 Caption: Gut microbiome. Found to be linked to the immune system. Photo: Shutterstock
Caption: Gut microbiome. Found to be linked to the immune system. Photo: Shutterstock
Are there collaborations at the Dangoor Center between researchers from different fields who try to inspire one another?
“Absolutely. For example, our collaboration with the researcher studying the microbiome. We have to find ways to break new ground, and to do that we need to join forces. Sometimes we might discover something that isn’t directly relevant to our own work but turns out to be a breakthrough for another researcher, exactly the missing piece they needed.”
Who are your research and development partners in Israel and abroad?
“It’s constantly evolving. For instance, in our fascinating study on the physical forces that affect the immune system, we collaborated with a leading American team from the University of Pennsylvania. We also work closely with clinicians from Sheba and Beilinson hospitals. We receive patient samples and study chronic inflammatory diseases. There are mechanisms we’ve developed that we’re now trying to adapt to other illnesses.”
Living the Dream, but Not Without Frustrations
Let’s talk a bit about your personal journey. What kind of environment did you grow up in as a child? Were you encouraged to study science and become a researcher?
“My parents strongly encouraged us, the children, to pursue an education. I remember myself as a very curious child, flipping through encyclopedias at home, searching for entries related to science. I wanted to know more, to understand how things work. For my parents, education was one of the most important values. My mother still says that the day I received my doctorate was the happiest day of her life.”
Why did you choose to specialize in immunology? When did you know it was your calling?
“As early as my undergraduate studies, I started looking for a lab for my internship and approached an immunology lab. The field fascinated me from the start, and over time it became one of the hottest areas in science. There’s no shortage of work for us, that’s for sure.”
How did you become involved in cancer research?
“Back in the United States, during my postdoctoral studies, I worked on cancer-related models, and when I came here and established my own lab, it was clear to me that this would be my focus. I’ve integrated other areas as well, such as congenital immune deficiencies, but the main theme has always been cancer.”
The academic world is still relatively male-dominated, especially at the senior faculty level. Do you feel that as a researcher?
“At the conference in Vienna, for example, dozens of scientists were invited to a gathering of Europe’s leading immunologists, and unfortunately, there were very few women there. Personally, it doesn’t bother me. There have been many times I’ve walked into a meeting and was the only woman in the room, yet I never felt inferior.
I have to give credit to Bar-Ilan University. Gender equity is taken very seriously here, and I think women’s involvement, including in leadership positions, is exceptionally high. A clear example of that is the number of female deans within the institution.”
Take us forward in time. What will the future of cancer treatment look like?
“Some diseases that we won’t be able to cure completely will become chronic, much like certain types of blood cancer. Just as a person with high blood pressure or high cholesterol takes medication, we’ll prescribe drugs for a certain period, or even for life, and the patient will remain stable. The disease will be controlled, and survival will not be compromised.”
Do you think people will live longer?
“Even now, we’re living longer than we used to, and there’s no doubt that’s where things are headed.”
What other dreams would you like to fulfill as a scientist?
“I come to work every day with enormous motivation and truly enjoy what I do. When one of our concepts turns out to be correct, that’s the greatest joy there is. Professionally, I’m living the dream.”
Your work has a profound impact on the lives of many people waiting for the treatments you’re developing. How do you handle that kind of responsibility?
“It’s not easy. I develop strategies in the lab, but it takes years before they reach the patient’s bedside because of regulations and other hurdles. During COVID-19, things moved much faster, and I can’t help but wonder if it’s possible to accelerate the process now as well. I see people around me suffering from these terrible diseases, and for now, we still don’t have solutions. There are so many promising ideas that work beautifully in mice but still don’t cure humans, and that creates a lot of frustration. I’m living the dream, but not without frustrations or self-criticism. That’s part of it.”
Do you have children?
“Yes, I have a son.”
Is he following in your footsteps?
“I often complain that he takes after us too much; he’s very dedicated to his work. He’s married, works in high-tech like his father, and manages a very large team that was recently acquired by Palo Alto Networks. So as a mother, thank God, I have plenty of pride and joy.”
Last Updated Date : 05/01/2026