Next-generation Immunotherapy – Unleashing Natural Killer (NK) Cells
Our immune system usually copes well with threats like bacteria, viruses, fungi, and even body cells that have unnecessarily begun to divide as a result of a DNA mutation. Why, then, is our immune system failing to cope with cancer?
One of the causes of our immune system’s inability to effectively respond to cancer is the ability of cancer cells to manipulate their surrounding environment in a way that suppresses immune response or utilizes techniques that allow them to evade the immune system altogether. This is where immunotherapy steps in.
Immunotherapy: A Conceptual Breakthrough
Immunotherapy is a therapeutic approach that harnesses the patient's immune system to effectively respond to cancer and other diseases. Recent studies that manage to decipher the interactions through which cancer cells suppress the immune system, or evade it, have led to conceptual breakthroughs in the development of immunotherapeutic strategies.
In a groundbreaking study published in the EMBO Molecular Medicine Journal, immunotherapy laboratory scientists led by Prof. Mira Barda-Saad from the Faculty of Life Sciences at Bar-Ilan University sought to find a cancer-targeted treatment that would eliminate the suppression of the immune system’s "natural killer cells" and allow them to act more forcefully against cancerous tumors.
Natural Killer Cells – The Body's First Line of Defense
The immune system is the sum of biological processes that protect our bodies from disease-causing micro-organisms. The immune system consists of two intertwined sub-systems – the innate immune system, and the acquired immune system.
The innate immune system immediately attacks any foreign cell it recognizes based on general, not specific, pathogen-associated motifs.
The acquired immune system is designed to specifically target pathogens that have invaded the body and is also capable of remembering and identifying pathogens – but its response is not immediate.
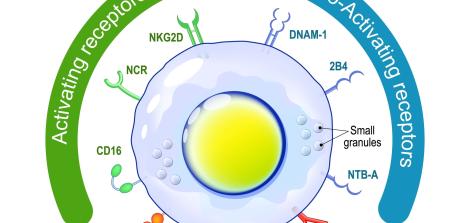
One of the types of white blood cells that are part of the innate immune system is natural killer cells (NK). These cells are lymphocytes that function within bodily tissues and travel in the peripheral bloodstream, and their function is to eliminate pathogens such as cancer cells and foreign micro-organisms such as viruses, bacteria, and parasites. Natural killer cells are the body's first line of defense from the moment of exposure to a foreign agent until its elimination or until the acquired immune system steps in.
The normal activity of NK cells depends on the balance between positive chemical signals that activate these cells and negative signals that inhibit their activity. When NK cells encounter cancer cells, the positive pathways are supposed to function and lead to the killing of cancer cells, and when NK cells encounter healthy body cells, the negative pathways are supposed to function and prevent cell damage.
In some cases, cancer cells are able to inhibit the normal function of NK cells. Previous studies have revealed how cancer cells do this: They secrete agents that inhibit NK cells' ability to attack cancer cells. The molecules that cancer cells secrete were identified as Cbl and Shp-1, (which are pronounced: "Sybil" and "Ship", respectively). One could say that activating Cbl and Shp-1"binds" the hands of natural killer cells and prevents them from operating at full power, thereby allowing cancer cells to thrive.
Research Challenges: Determining Which Mechanism to Use, How to Deliver It, and Proving It Is Effective and Safe
The purpose of this research was to find a way to unleash natural killer cells. In other words: Developing a preparation that will penetrate cancer cells and inhibit their ability to activate the Cbl and Shp-1 molecules, which inhibit the function of natural killer cells, or put simply: activating natural killer cells at full throttle by inhibiting the inhibitors.
Achieving this involves finding a solution to several challenges:
A. Finding the Mechanism that Inhibits the Secretion of the Inhibitors
The mechanism chosen was genetic inactivation of cancer cell genes responsible for secreting the Ship and Sybil molecules, using siRNA, "the RNA silencer". This molecule consists of a short sequence of nucleic acids (RNA) and is responsible for several functions, including interference with gene activity and gene inactivation. The role of these molecules was discovered in plants in 1999, and scientists have since learned to control and use them as targeted gene silencers.
In the present study, scientists specifically designed siRNA molecules to inactivate the genes responsible for the immune cell inhibitors Cbl and Shp-1 expression. – . In a cell trial, scientists confirmed that siRNA molecules are indeed effective in fulfilling their goal and are capable of silencing the Ship and Sybil secreting genes.
Various levels of siRNA doses were also tested to determine the most effective gene silencing dose, i.e., a dose that would be high enough to induce the desired response.
B. "Drug Delivery" to Targeted Cells Only
Next scientists sought to develop a treatment that will only target cancer cells – without inducing an immune response against the treatment itself. Another challenge posed by blood cancer is the fact cancer cells may spread throughout the body, hence targeting them is very difficult.
The chosen solution was using a Lipid Nanoparticle (LNP) capsule, an innovative mechanism used for the delivery of various drugs using tiny lipid particles, on a nanometric scale, for carrying active molecules to target organs. In order to bind to certain cells under specific conditions, the nanoparticle capsule can be designed to only bind to selected receptors in the targeted cells.
The main advantage of using this technology to carry and deliver the siRNA component to cancer cells was the composition of these particles, which allows them to travel in the bloodstream and target specific cells without inducing an immune response. Another advantage is that they are non-toxic and do not affect healthy cells.
The present study used an innovative form of lipid nanoparticles, which encapsulates the siRNA component delivered to natural killing cells. (Diagram A)
Coating of NK Antibodies
Once the proper mechanism for blood-borne drug delivery was found, scientists needed to design the nanoparticles to target only NK cells. The nanoparticle capsule was therefore coated with antibodies, which are proteins that have a unique structure, to give it the ability to bind to a specific receptor on the surface of NK cells. A possible analogy would be that these antibodies on the nanoparticle capsule serve as a key designed to open only one lock – the receptor on NK cells, without affecting any other cells.
The researchers then tested the effect of this preparation in cells (In Vitro) and proved that, as expected, this preparation fulfilled its function in NK cells without affecting other cells, thanks to the antibody coating, which allowed the preparation to bind to a specific receptor.
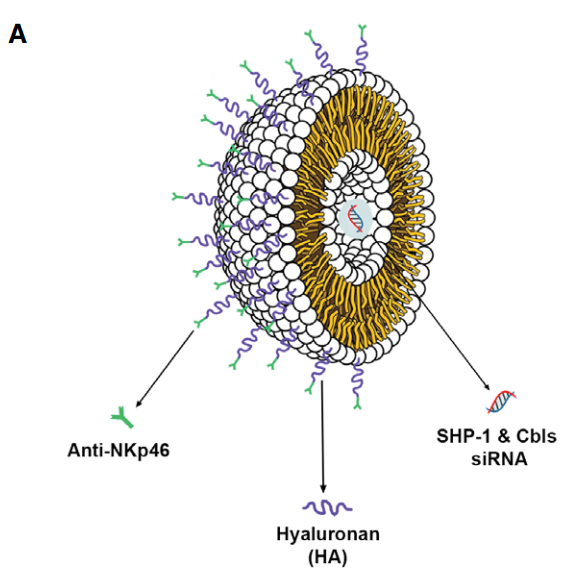 An illustration of the lipid nanoparticle capsule,
An illustration of the lipid nanoparticle capsule,the antibodies binding to NK cell receptors, and the siRNA molecule
C. Ensuring Minimal Harm to Healthy Cells and Organs
In order to confirm the preparation's safety and basic suitability for human treatment, the preparation was tested in a mice model. An active-siRNA carrying nanoparticle preparation was injected into mice transplanted with lymphatic human cancer cells, which had developed into cancerous tumors, while an inactive-siRNA carrying nanoparticle preparation was injected into other mice for comparison - the control group. Overall, the mice received six treatments.
The researchers examined the treatment's effect on tumor size and growth rate and also monitored the mice’s health. The researchers noted that mice treated with the new preparation showed no effects of weight loss, fatigue, or any other evidence of stress or injury as a result of the treatment – over a prolonged period of time from the beginning of the treatment.
At the end of the study, the mice were dissected, and the concentration of the preparation was examined in different body organs and in the cancerous tumors. Indeed, the concentration of the preparation was found to be high in the cancerous tumors, whereas in the mice’s hearts, lungs, and kidneys the concentrations of the product were low – indicating the researchers' successful development of a targeted treatment.
D. Proof of Concept
In the final part of the study, the researchers sought to evaluate the efficacy of the preparation in reducing the volume of cancerous tumors.
The treatment, as observed by the researchers, significantly reduced the size of cancerous tumors and their growth rate, and significantly prolonged the lifespan of the treated mice compared to the control group.
The Bottom Line
A combination of innovative and advanced technologies – such as siRNA mechanisms, a lipid nanoparticle capsule, and engineered antibodies – has led to the development of a targeted preparation capable of blocking immune suppressors and harnessing the powerful NK cells to fight cancer cells. In the future, the researchers hope, these innovative therapies will constitute an effective and safe treatment for various cancer types and other diseases.
Last Updated Date : 13/03/2023