Dr. Nitzan Gonen created testicles in the lab and opened a giant gateway for future research - Interview
One of the highlights of pregnancy is the moment when the baby's gender is discovered. Tension and excitement build up within the family as they wait for the big "reveal" in the 13th week of pregnancy. But why can the gender of the fetus only be known at that point, rather than immediately after conception?
Dr. Nitzan Gonen from the Dangoor Center for Personalized Medicine at Bar-Ilan University is a biologist who researches sex development in embryos – a complex process during which many things can go wrong. Recently, as part of her research, she succeeded in creating “mini-testicles” under laboratory conditions from mouse testicle cells, scientifically referred to as a “testicular organoid.” This scientific breakthrough has received significant attention in newspapers in Israel and around the world.

Not everything happens in coding genes: The discovery that launched her career
Dr. Gonen showed an interest in biology from a young age. Coincidentally, her then-boyfriend, who later became her husband, is the son of her high-school biology teacher. After completing her bachelor's, master's and doctoral degrees at the Technion, she continued on to postdoctoral research at the Francis Crick Institute in London, Europe's largest biomedical research center. In 2019, she returned to Israel and was shortly thereafter selected as one of The Marker’s 40 most promising young people. Today, her lab in the department of Life Sciences at Bar-Ilan university is a part of the Dangoor Center for Personalized Medicine. In 2023, she won the prestigious Krill Prize for excellence in scientific research from the Wolf Foundation.
Dr. Gonen's area of expertise is the determination of biological sex in embryos and sex reversals. Although biological sex in humans is essentially determined at the moment of conception, it takes about seven weeks before the differences between male and female embryos begin to manifest. Until then, male and female embryos are completely identical. This same process occurs in mice around the tenth day of pregnancy, and they serve as animal models for research in this field.
Things don't always go smoothly. One in 4,000 babies is born with a discrepancy between the genetic sex determined at conception and the reproductive system or organs that develop during pregnancy. These cases are medically termed Disorders of Sex Development (DSD). In extreme cases, there is a condition of sex reversal- where a baby is born with male XY chromosomes but appears entirely female, or vice versa.
Before Dr. Nitzan Gonen's research, science was aware of over 100 mutations in the genes responsible for determining sex that cause sex reversal in humans and mice. Yet, in more than half of the cases, when trying to determine what went wrong in development, the cause could not be found. During her postdoctoral research, Dr. Gonen found a piece of DNA that was not within a gene at all- when she removed it, she observed a complete sex reversal from male to female.
How do you explain this?
“The approach in genetics is that there is a certain order in the bases of genes, and if there is a mutation and that order is disrupted, it causes problems. In other words, if you fix the defective gene causing the sex reversal, it will solve the problem. The thing is, only about 2% of the DNA strands are genes. 98% of DNA are not genes; they have other functions. Among other things, parts of them are responsible for gene regulation, like on and off switches, and they make sure that genes are expressed at the right time, in the right place and in the right amount. The piece of DNA I discovered in my research is of this type, and it enhances the process of gene expression simply by being present. This discovery caused me, my colleagues in the field of sex reversal, and geneticists in general, to realize that mutations will not always be located in the 2% of the genome related to sex determination. The rest of the genome is also important.”
Personalized diagnosis: The first step in personalized treatment
Dr. Gonen's groundbreaking discovery was published in the scientific journal Science and launched her career, but she did not stop there. She had a hunch that this piece of DNA was just one of many pieces with a similar role. Today, from her lab at Bar-Ilan, she collaborates with teams from Australia and France who have a huge database containing the genetic information of undiagnosed individuals with DSD. Together, they are determined to map all of the other pieces. “We are looking for all of the elements in the DNA within the 98% that are not genes, which are similar to the piece we found and are involved in the process of sex determination.”
Isn’t searching through 98% of the genome like looking for a needle in a haystack?
“We managed to narrow the search field from 98% of DNA to 3.7% of DNA, which means about a hundred different options that need to be checked for each person, instead of four million. We are going to publish an article soon on one of our new findings. Here’s a spoiler: We found a piece of DNA that we suspected was important, and when we removed it, female mice were born without a female reproductive system at all. No ovaries, no fallopian tubes and no uterus.”
How much does this shorten the search?
“Today, within two weeks, you can get a full genetic profile, but even when you have the information within a relatively short time and at a reasonable cost, these children lack a diagnosis because the cause of their problem is unknown. The goal is to collect information about all of the elements that might affect sex development and eventually bring the findings back to the clinic, so that doctors can provide a diagnosis to their patients after a full genetic test. Being able to diagnose is very important. When a person has a disease and they undergo many tests, but no one can tell them what’s wrong with them, the lack of knowledge can drive them crazy.”
Diagnosis is indeed important, but is there a treatment for DSD disorders?
“When it comes to children with sex reversal, especially in extreme cases where the sex cannot be determined based on external reproductive organs, parents sometimes determine the sex for them at an early stage. Although there is an option to register these children as genderless on their identity card, in practice, our society is not built to handle this complexity. According to the sex chosen by the parents, the child will receive sex-matching treatments like plastic surgeries and hormonal treatments from a young age. The problem is that when the cause of the problem in the fetus’ sex development is unknown, wrong decisions may be made when choosing the sex.”
What kind of wrong decision can parents make, for example?
“Biological sex is not always the sex that should be chosen. If, for instance, there is a hormonal problem of testosterone absorption, then hormonal treatments with testosterone won't help, even if the chromosomes indicate male biological sex. It is definitely not advisable to wait until the child reaches adolescence to discover that this is the case. Here lies the importance of early genetic diagnosis and personalized medicine, which can facilitate appropriate treatment and prevent unnecessary suffering.”
Is it possible to prevent sex development disorders from the start?
“I hope that future research will enable the screening of embryos that do not contain these mutations in pre-implantation genetic tests (PGD), which are done after in vitro fertilization and before they are returned to the woman’s body. This technology exists today at all hospitals and in vitro fertilization clinics. Currently, there are about 600 different genetic diseases that can be identified in advance, and in the future, we will be able to add DSD disorders to the list.”
Testicles in a laboratory dish: Can we cure infertility?
Sex determination research faces significant challenges. In humans, these problems arise from limited access to embryos at a critical stage of sex development. In humans, this window is the 7th week of pregnancy, a stage when the embryo is very small, making it difficult to obtain enough cells for research. In mice, the critical window is between the 10.5th day and the 15th day of pregnancy. Even with mice, embryonic tissue is very small during this period.
To overcome these challenges, a new idea was developed – to create organoids. Organoids are miniature, three-dimensional models of organs that can be studied freely in the lab. One method of creating organoids is from programmed stem cells- cells taken from any part of the body and adapted to create the desired model. Dr. Gonen set a goal to “program” ordinary cells so that they develop into embryonic testicle cells. A similar idea was implemented in Japan on an ovary organoid.
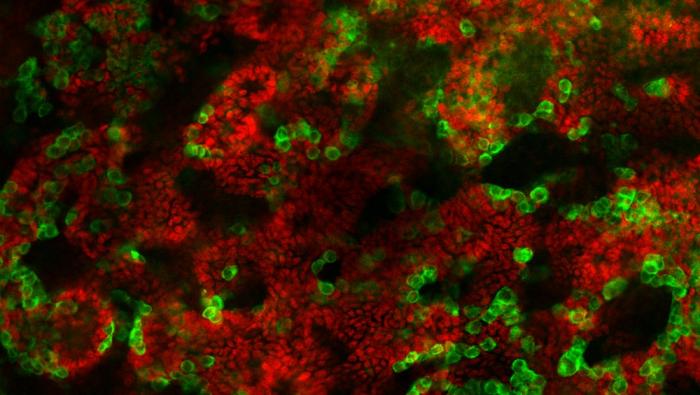
In the lab, Dr. Gonen and her team took cells from the testicles of young mice, broke them down into individual cells, and reassembled them in a solution containing the essential substances for cell growth that are present in the testicle. After many attempts, they managed to create a “mini testicle” in a dish which was maintained for nine weeks – a significant duration compared to original cells, which survive for less than a week.
What is the significance of this achievement for your research on sex determination?
“It was found that the testicular cells in the organoid are organized in a manner that is very similar to a real testicle, including the creation of tubes that transport the sperm cells within them. During this process, researchers learned a lot from the mouse testicle cells: what are the exact conditions required to keep these cells alive, and which substances and what environment they need. With this knowledge, they hope to create a suitable programmed testicle organoid in the future. This achievement will allow us to study the complex processes of sex determination and sex development disorders and also learn about the formation of sperm cells in a much more accessible and controlled way.”
Now, the big question is: Can the mini testicle produce sperm?
“In the lab, we managed to 'mature' the organoid to the stage where sperm begins to form, and we have initial evidence that it can start the process, which takes about five weeks in mice. We are conducting various experiments to understand how much sperm is produced, what stage the process can reach, whether the sperm is functional and whether it can create an embryo. The sperm can be functional even if the process of sperm cell creation in the testicle is not fully completed.”
What practical applications could this discovery have?
“One example is preserving the fertility of children with cancer. The sperm cells of children with cancer are often damaged by chemotherapy treatments. In 50% of cases, the young sperm cells of the children will be destroyed, and they will remain infertile. In children, the destruction of the cells occurs before the sperm cells have had a chance to mature, and there is currently no way to preserve their fertility. We have a collaboration with Hadassah Ein Kerem Hospital, aiming to take a biopsy from the child's testicle before starting chemotherapy, and, similar to what we did with mice, break down the samples into cells and reassemble them. We will test if it is possible to mature these models in the lab and begin the process of sperm production.”
And what about curing infertility in general?
“The World Health Organization found that one in six couples in the world will experience infertility. In about half of the cases, infertility is due to a problem with the male. Today, there are medical solutions for infertility when it is caused by low sperm count, abnormal sperm structure or blockages in the sperm duct, but there is one problem that has no solution: men whose testicles do not produce sperm at all. In the distant future, we might be able to take a cell from a man, such as a skin cell that contains his DNA, and then program it into testicle cells and sex cells to produce sperm in his own testicular organoid.”
Personalized medicine in blue and white
Dr. Gonen refers to her postdoctoral period in London as “the seven good years.” She worked under Dr. Robin Lovell-Badge, who discovered the gene that initiates the process of testicle formation in the embryo. This researcher was himself a student of Dr. Martin Evans, who won the Nobel Prize in 1981 for discovering embryonic stem cells. For Dr. Gonen, this period was extremely productive both professionally and personally- her two younger daughters were born in London. However, at the end of the period, she decided to return to Israel, despite having the option to stay.
How was it to return from London to Israel and why specifically Bar-Ilan?
“Returning to Israel was challenging. We returned directly to COVID-19, and before all my lab equipment arrived, we went into lockdown- but the homely feeling in Israel remained. The children speak Hebrew, and now we are close to their grandparents. At Bar-Ilan University, I found supportive and friendly people, and that is scientifically important. A research role requires commitment, so it was important for me to choose a comfortable and pleasant place to work, and Bar-Ilan proved to be the right place.”
Are there advantages to academia in Israel?
“Absolutely. There is something special about Israeli culture that you don’t find elsewhere. The friendliness and mutual assistance create an open and stimulating environment, enriching research and collaborations. I am happy to contribute my small part, do my research here, and train the future scientists of Israel.”
Main image credit: Avia Stoppel
Last Updated Date : 12/11/2024