Innovative Immunotherapeutic Strategy in Blood Cancer Treatment: Targeting Cancer Cytoskeletal Proteins
Blood cancers are a group of severe cancer types that every year afflict more than 1.2 million people around the world, with an annual death toll of more than 700,000. A new study led by Prof. Mira Barda-Saad of the Immunotherapy Laboratory at the Bar-Ilan Faculty of Life Sciences proposes a new immunotherapeutic strategy for the treatment of blood cancer, adding another ray of hope for people suffering from this disease.
Blood Cancer: Existing Treatment Methods
In leukemia, blood cells begin to divide and multiply uncontrollably and harm healthy cells in the circulatory system, while evading the immune system, whose primary function is to combat such threats.
Despite advances in cancer-targeted therapy in recent years, current treatment strategies for blood cancer still revolve around chemotherapy and radiotherapy, which damage the bone marrow, concomitantly with stem-cell (bone marrow) transplantation therapy designed to help the blood and immune systems recover.
One of the key problems with such treatments is that they damage not only cancer cells but also healthy cells, and therefore affect many systems in the body. Consequently, these treatments have side effects that significantly compromise patients' quality of life.
The main challenge facing cancer researchers and scientists today is therefore developing "targeted" therapies capable of focusing on unique molecular structures and fighting only cancer cells without harming healthy cells or interfering with their functions.
In this study, led by Prof. Mira Barda-Saad a new immunotherapeutic strategy focuses on a protein known as WASp (Wiskott-Aldrich Syndrome protein), which has a unique structure and can be found in the skeleton of blood cancer cells (cytoskeleton). Given their in-depth understanding of human cellular mechanisms and using artificial intelligence and machine learning capabilities, researchers were able to identify small molecule compounds capable of inhibiting only this unique protein structure and not any other structure that exists in other cells.
Cancer Cytoskeleton: What Is the WASp Protein?
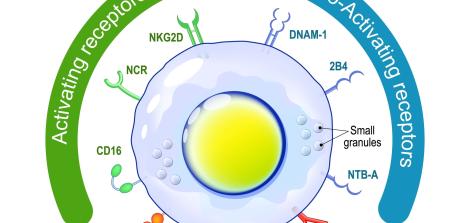
A healthy cell has a stable skeleton – a dynamic structure made of protein fibers that gives the cell its shape and has several important functions, including cell proliferation and metabolism. The WASp protein is used to produce and shape actin, one of the main skeletal proteins, and therefore plays a crucial role in normal cellular functions.
Although the role of WASp in carcinogenesis is not yet fully understood by scientists, researchers have discovered that in cancer cells, this protein maintains a unique structural state, which they called the "open" structural state. This state makes blood cells stop performing their normal functions and start reproducing uncontrollably.
A previous study even demonstrated that this unique characteristic might be the weak point of cancer cells and can therefore be targeted by drug therapy. The current study demonstrated that targeting the WASp protein in its open structural state and causing its degradation will cause cancer cells to die.
The next and crucial step of development was finding a molecule that can identify the cells in which the WASp is in an open structural state and lead to their destruction without harming other cells. This is the purpose of the current research.
Development Process: Identifying a Small Molecule Compound
90% of all medicinal preparations are made of small molecule compounds or SMCs. These tiny low molecular weight organic compounds can affect biological processes and are used, among other things, in targeted cancer treatments.
The purpose of the study was to identify an SMC on which the drug mechanism could be based so it would bind only to the "open" rather than the "closed" WASp protein and cause its degradation, leading to the breakdown and death of the entire cancer cell.
The challenge: To date, approximately 230,000 SMCs have been identified and mapped and are available to drug developers. How do we find out which SMCs can potentially be suitable for the task? Such tasks were almost insurmountable, until the introduction of artificial intelligence and machine learning into research and science.
As part of this study, a computerized detection (In Silico) process was performed using artificial intelligence and machine learning capabilities. This process involved the use of technology that simulates the function of these molecules within biological mechanisms, thereby helping researchers focus on several dozen out of thousands of these molecules. These molecules were used for the next step – laboratory research of cancer cell cultures.
Cell (In Vitro) Research: Finding the Right Molecule
In order to confirm computer modeling projections and find a molecule that will be most effective and will focus on the "open" WASp without affecting healthy cells in which the WASp is "closed", the researchers examined the effects of the selected molecules on cells in the laboratory.
Out of the dozens of molecules tested in this manner, the researchers identified a single molecule, nicknamed SMC #13, that was able to bind to the receptor of the "open" WASp, disrupt its activity, and cause this protein to break down.
Apart from its effects on cancer cells, the researchers also verified that this molecule does not bind to other proteins and does not interfere with the normal functions of healthy cells in which the WASp protein is “closed” rather than “open”, so it could be used as a targeted treatment without endangering healthy cells.
Animal Model (In Vivo) Research: Safety and Efficacy
The next step in the process was research in animal models to test the safety and efficacy of the chosen molecule. As part of the study, the scientists injected the SMC #13 molecule into laboratory mice carrying human blood cancer (non-Hodgkin lymphoma), and saw that indeed, the treatment reduced tumor size, stopped disease progression, and prolonged the mice’s lifespan without harming other healthy cells. In doing so, the researchers demonstrated that the molecule they had chosen is capable of targeting the cytoskeletal protein, WASp, in its open state, and causing it to break down, thereby killing the cancer cells, without damaging healthy cells or other organs.
Therefore, the scientists concluded that this treatment constitutes a novel immunotherapeutic strategy for targeting the cancer cytoskeleton. The study findings make the SMC #13 molecule a potential candidate for clinical development in humans as well.
The Basis of the Research: An In-depth Understanding of Cellular Mechanisms Combined with Machine Learning Capabilities
The treatment strategy targeting the cancer cytoskeleton and the WASp protein was developed based on two principles that guide the laboratory staff led by Prof. Mira Barda-Saad: The first is having an in-depth understanding of cellular mechanisms, which contributed to the discovery of the role played by the WASp protein, including its unique structure in cancer cells; the second is using interdisciplinary knowledge, including computerized predictive tools developed by Prof. Yanay Ofran, from the Institute of Nanotechnology at Bar-Ilan University.
These principles allow the laboratory to open new avenues for the development of targeted therapies, not just for laboratory scientists, but also for other researchers and scientists, thereby promoting the field of targeted cancer treatments as a whole.
Last Updated Date : 13/03/2023