Has the Mechanism that Causes Inflammatory Bowel Diseases been Deciphered?
Goblet cells are intestinal cells that secrete mucus, creating a barrier between the intestinal microbiome (ecological community of microorganisms) and the intestine itself. Intestinal mucus plays a crucial role in the interaction between the host and its microbiota. Damage to it is a hallmark, and possibly the cause, of a variety of inflammatory bowel diseases (IBD) and enteric infections. Despite the importance of the mucus layer, it remains unclear how goblet cells control the amount of mucus they secrete into the gut. Dr. Shai Bel from the Azrieli Faculty of Medicine at Bar-Ilan University headed a group of researchers seeking to understand how this process is regulated.
Autophagy is an intracellular process responsible for recycling cellular components, which is activated in times of stress such as starvation, infection, and accumulation of unfolded proteins in the endoplasmic reticulum (ER). Since mutations in autophagy-related genes are a risk factor for IBD development, the researchers decided to investigate whether autophagy is necessary for the proper goblet cells' function.
First, the researchers wanted to test the hypothesis that enhanced autophagy will lead to higher mucus secretion by goblet cells. Hence, the researchers worked with mutant mice named Becn1F121A. In these mice, the autophagy process is constantly active, due to a mutation in the gene that codes for the Beclin1 protein that plays a critical role in the autophagy regulation. Indeed, the mucus layer in Becn1F121A mice was thicker than in mice without the mutation (wild type). In addition, lower levels of bacterial-origin proteins were found in the blood of these mutants, and the expression of genes involved in anti-bacterial response was dampened. The gut of the mutant mice is, therefore, better protected against the penetration of immunogenic factors compared to mice without the mutation.
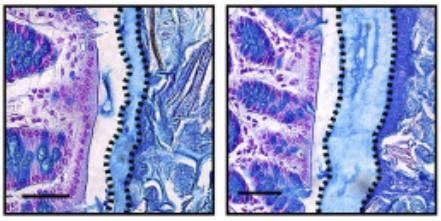 In the figure colonic tissue section microscope image - on the left, wild-type mouse (without mutation) tissue, on the right, Becn1F121A mutant tissue. The mucus layer is defined by the dashed line.
In the figure colonic tissue section microscope image - on the left, wild-type mouse (without mutation) tissue, on the right, Becn1F121A mutant tissue. The mucus layer is defined by the dashed line.
After the researchers saw that autophagy plays a role in gut mucus secretion, they sought to understand the mechanism by which autophagy activation promotes mucus secretion. The researchers first tested whether the Becn1F121A mice have more goblet cells and therefore secrete more mucus, but experiments showed a slight reduction in the number of goblet cells in mutant mice. Next, they hypothesized that mutant mice overexpress mucus-forming genes, but this hypothesis was also found to be false. The hypothesis suggesting that there is a change in reactive oxygen species levels (ROS), which are known to be affected by autophagy, which impacts mucus secretion in the mutant mice's goblet cells was also ruled out. Finally, researchers noticed a difference in the ER stress markers between the Becn1F121A mutant mice and wild-type mice, with reduced ER stress was observed in the goblet cells of the mutant mice. To understand the connection between ER stress and mucus secretion, the researchers performed the following experiments -
- Becn1F121A mice were treated with ER stress inducer thapsigargin, and a decrease in the mucus layer thickness was observed.
- Wild-type mice were treated with TUDCA, a chemical that reduces ER stress, and an increase in the mucus layer thickness was observed.
In addition, the researchers found that in Bcl2AAA mutant mice, mutants with suppressed autophagy, there is a significant decrease in the thickness of the mucus layer and even a complete absence of mucus in some regions.
All the results together led the researchers to the conclusion that autophagy controls ER stress and that ER stress is a cell-intrinsic switch that controls mucus secretion from goblet cells.
Does the microbiome play a role in the mucus secretion in the gut? To answer this question, researchers treated germ-free mice, and normal mice that were microbiota-depleted due to intensive antibiotic treatment, with ER stress reducer TUDCA. In both cases, no increase in mucus layer thickness was detected. In addition, mutant mice lacking Nod2 gene, a gene that codes for the intracellular sensor of bacteria and is known to be Crohn’s-disease-risk gene, showed no response to TUDCA treatment. These results show that the ER-stress-mediated regulation of mucus secretion is microbiota dependent and requires the bacterial sensor, the Nod2 protein.
And what about the effect of excess mucus secretion on the microbiome? To analyze the composition of the gut microbiome, fecal samples were taken from normal mice (wild type) and Becn1F121A mutants. 16S ribosomal RNA gene sequencing revealed a difference in the microbiome composition between the two types of mice. In mutant mice, the gut bacteria population was characterized by higher microbial diversity, and the mucus-utilizing bacterium Akkermansia muciniphila was abnormally over-abundant. Given that factors such as mouse housing conditions and diet have a great influence on the microbiome composition, the same analysis was carried out on mice feces from an animal facility in the USA, and similar results were obtained.
Finally, the researchers wanted to understand whether the Becn1F121A mutants are better protected against inflammatory and infectious intestinal diseases compared to normal mice. The answer was yes; researchers treated mice with dextran sulfate sodium, which is known to instigate colonic inflammation, and following the treatment mutants developed less severe inflammation symptoms compared to normal mice. Furthermore, even in the case of infection with bacteria that is strongly associated with IBD, Becn1F121A mutants did not develop inflammation, in contrast to wild-type mice.
Another interesting discovery revealed that the microbiota provides only partial protection from colitis. Becn1F121A mice microbiome was "transplanted" into germ-free mice by fecal transplantation and the mice were treated with dextran sulfate sodium. Although the transplanted mice developed intestinal inflammation symptoms, the intestinal tissue damage was more moderate compared to a control group - mice that were "transplanted" with a normal mice microbiome.
In conclusion, Dr. Shai Bel and his colleagues demonstrated that autophagy regulates mucus secretion from goblet cells by alleviating endoplasmic reticulum (ER) stress in a microbiota-dependent manner. Their results provide a possible explanation for why IBD patients experience a high frequency of mutations in autophagy-related genes. A better understanding of how intestinal mucus secretion is regulated will allow researchers and physicians to find a way to help and possibly even cure patients suffering from a variety of IBD.
This work was published in the prestigious journal "Cell Host & Microbe".
Last Updated Date : 12/11/2024