A Cure for a Rare Genetic Disorder? The Application of CRISPR Technology Proves This Is Possible
A new groundbreaking study led by Dr. Ayal Hendel from the Mina and Everard Goodman Faculty of Life Sciences at Bar-Ilan University, in collaboration with researchers from Tel Aviv University and senior physicians from Sheba Hospital in Tel Hashomer, suggests an innovative genetic treatment strategy for a severe genetic disorder – Severe Combined Immunodeficiency (SCID), also known as "bubble boy disease".
SCID
SCID is a group of diseases which are characterized by a profound block in the development of T-cells, a certain type of white blood cells, which severely harms the immune system's ability to respond to pathogens. Some SCID diseases also impair the development of B cells and natural killer (NK) cells. One of the measures taken in the past to protect children with this disease was keeping them inside a sealed bubble that protected them from bacteria and other pathogens, which led to the disease being commonly called "bubble boy disease".
In Israel, the incidence of SCID is estimated at 1 in every 29,000 births. This incidence rate is particularly high – for example, in theUnited States, it is estimated to occur in about 1 in every 58,000 births. The reason for this is a high rate of consanguineous marriages, which increases the chance of inheriting the defective gene that causes the disease from both parents.
SCID diseases are monogenic - meaning they are caused by a defect in one gene and can be inherited in a variety of ways. Babies born with SCID may be healthy for the first few weeks of their lives but exposure to environmental pathogens and the decline of antibodies transferred from their mothers make these babies vulnerable to life-threatening bacterial, viral or fungal infections. In the absence of medical intervention to restore the immune function, many babies with this disease die before reaching the age of two.
The standard-of-care treatment and its disadvantages
The standard-of-care for SCID is bone-marrow transplantation. This means the patient is transplanted with healthy progenitor cells capable of producing white blood cells in order to restore immune functionality. Complete genetic compatibility between the patient and the donor can raise the treatment efficacy rate to as high as 90%, while partial genetic compatibility may reduce the treatment success rate to only 60%-80%. The reasons for failure range from poor reception of healthy cells in the patient's body to the onset of serious diseases resulting from the patient’s cells being attacked by the transplanted cells.
Given the limitations of bone marrow transplantation, the difficulty of guaranteeing complete genetic compatibility between the donor and the patient, limited success rates, and potential side effects, there is a strong need to develop alternative treatments for SCID.
The objective: A CRISPR-based genetic treatment
The current study examined one of the most advanced alternative treatments: genetic engineering of progenitor cells from the patient's own blood system aimed at correcting their genetic code and thus curing the disease. As opposed to transplanting bone marrow from a donor, in this case the patient himself is the donor. This significantly reduces the risks involved in the transplantation of bone marrow from another person because it diminishes the risk of transplant rejection and the triggering of an immune response.
While over 20 genetic defects are known to cause SCID, in this study, the researchers focused on one genetic defect caused by a mutation in RAG2 genes. The study was intended to extract progenitor cells from the patient's body, genetically edit them and repair their damaged genes - then return them to the patient’s body.
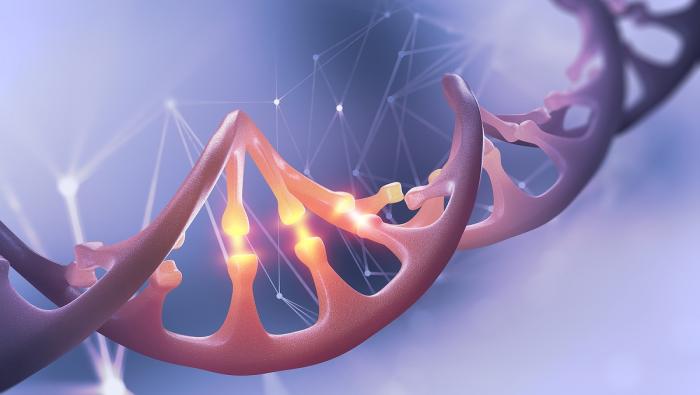
To genetically edit the patient's progenitor cells, their damaged DNA section had to be replaced with a new, normal section. For this purpose, the researchers had to cut the DNA strands at the location of the defective section and replace it with a corrected section. CRISPR technology enabled them to perform the following actions:
1. Cut the defective section: the targeted section is cut with the help of an RNA component capable of binding to a specific region of the gene and a "Cas9" protein, which enables accurate cutting of the DNA at the targeted location. This can be described as using pre-engineered "nano-scissors" capable of cutting genes at a specific location.
2. Insert a normal section: in addition to cutting the defective section, scientists inserted into the cell a corrected DNA sequence of the same gene, which had been engineered based on a healthy cell and was carried by a vector of an adeno-associated virus (rAAV6). In order to insert the CRISPR system into the cell nucleus, the researchers performed what is known as electroporation - the controlled use of electric current to temporarily create small pores in cell membranes through which the CRISPR system can enter the cells.
3. Fuse the normal section: at this point the cell's genetic repair systems kick in, fusing the cut DNA strands with the foreign DNA introduced into the cell.
The results of the experiment
The researchers were able to repair the RAG2 gene in immune progenitor cells taken from a specific patient, thereby proving their hypothesis that cells unable to develop
T cells because of a defective section regain this ability after this particular section is replaced.
The study proved the feasibility of using the system to genetically edit blood cells in SCID patients, which paves the way toward developing similar treatments for other genetic blood disorders. This technology is not yet in clinical use, as the repaired cells have not yet been returned to the patient's body, but in the coming years, scientists hope it will become possible to use CRISPR technology to effectively and safely cure SCID and other diseases.
News and Announcements
Last Updated Date : 29/12/2022